|
 |
- Search
| Arch Hand Microsurg > Volume 28(4); 2023 > Article |
|
Abstract
Purpose
Ablation of malignant neoplasms of the scalp usually leaves extensive defects. Free musculocutaneous transfer using the latissimus dorsi muscle with a skin graft has been the gold standard for restoring such defects; however, this approach may be limited by potential functional morbidity and delayed healing of the grafted skin. This study presents a case series of microsurgical reconstruction with thoracodorsal artery perforator (TDAP) flaps for extensive scalp defects after radical oncologic resection.
Methods
Consecutive patients with malignant scalp tumors who underwent wide local excision and defect coverage with TDAP free flaps between April 2019 and January 2023 were evaluated. Operation-related data and surgical outcomes, including donor- and recipient-site complications, postoperative shoulder function, and aesthetic results, were thoroughly assessed. As an oncologic outcome, local recurrence was also recorded.
Results
Eleven patients who underwent reconstruction with an average flap size of 223.2 cm2 (range, 160.0–406.0 cm2) were identified. The flaps were based on an average of 2.4 perforators. The donor-site wound was closed primarily in all cases. All flaps achieved complete wound healing within an average of 2 weeks and presented stable resurfacing of the scalp with satisfactory morphological outcomes. No functional shoulder disability was observed in the postoperative period. Five patients exhibited local recurrence but were appropriately treated in a timely manner.
Scalp defects may be caused by trauma, infections, tumors, congenital malformations, and neurosurgical interventions, among other reasons. The size and depth of excision would determine the reconstruction procedure: for defects with a small-to-moderate size and a shallow raw surface, the primary approach involves transfer of skin grafts or local flaps with guaranteed perfusion [1,2]. However, malignant neoplasms of the scalp require a certain extent of resection with safety margins along the tumor perimeter; this results in extensive, bony, and deep defects [3]. In such cases, microsurgical reconstruction with a free tissue transfer has been the first choice for preserving the recipient-site structure and function and for ensuring aesthetic outcomes, even though it is technically more difficult and challenging than alternatives such as skin grafting and local flap transfer [4-8].
For extensive scalp defects, the commonly proposed free flaps include the latissimus dorsi (LD) musculocutaneous flap (with or without a skin graft), the anterolateral thigh (ALT) fasciocutaneous flap, and other flaps for elevation with large soft tissues [5,9-11]. A free LD musculocutaneous flap covered with grafted skin is one of the most popular options for resurfacing large scalp defects [12-14]. This technique is appealing because of a well-vascularized bed, acceptable graft texture, and wide soft tissue coverage. However, a prolonged skin graft take, need for a graft donor, and donor-site morbidity of decreased shoulder function due to extensive muscle resection have been noted as disadvantages [15]. While ALT free flaps are reliable alternatives, only flaps of limited sizes can be harvested, and skin grafting is still required for larger defects [9].
Since it was first reported by Angrigiani et al. [16], thoracodorsal artery perforator (TDAP) free flaps have been utilized in recent years. Their strengths include the presence of a reliable perforator anatomy, provision of generous skin dimension, long pedicle length, and minimal functional morbidity [16-19]. These advantages may allow for favorable aesthetic and functional outcomes at both the donor and recipient sites. Nevertheless, there remains a paucity of literature on their use and outcomes for extensive scalp defect. Thus, in this retrospective study, we aimed to present a case series of reconstructions of extensive scalp defects with TDAP free flaps following tumor resection and review our experiences by evaluating the case outcomes.
Ethics statement: This study was approved from the Institutional Review Board (IRB) of Samsung Medical Center (No. 2023-07-150) The study was performed in accordance with the Declaration of Helsinki, and written informed consent was obtained for the publication of this article, including all clinical photographs.
This was a single-center retrospective review of cases of open wound coverage using TDAP free flaps after wide local excision of malignant scalp neoplasms between April 2019 and January 2023. Patients with at least 6 months of follow-up after surgery were included. Cases with missing or unreliable data were excluded. All surgical procedures were conducted by the senior author (KTL).
Under general anesthesia, the patients were placed in the lateral decubitus position with the scalp lesion and back in the same operative field. After wide local excision with safety margins set according to the tumor type and nature [20,21], all specimens were processed for frozen section analysis; further resection was performed based on the analysis findings. In all cases, the tumor did not invade or extend beyond periosteum; thus, the excision was confined to the depth of the subperiosteal layer. If a suspicious lesion was observed in the periosteum or calvarium during the ablation, additional burring of the outer cortical bone was performed while leaving the dura mater intact.
Once the final defect size and depth were determined, the TDAP flap was designed and harvested following a protocol previously described by our institution [17,22]. Intraoperative handheld audible Doppler or high-frequency ultrasonography was used for perforator mapping, and the skin paddle was designed as a transverse ellipse to include both the descending and transverse branches of the thoracodorsal artery (as the dimension allowed). The elevation plane was determined according to the requirements of defect reconstruction, taking into account the contour and shape of adjacent tissue around the defects, and the amount of dead space. When harvesting multiple perforators from both branches of thoracodorsal artery, the muscle intervening these branches was transected and repaired. LD muscle was spared in all instances and drains were placed at the donor site.
After obtaining IRB approval, the medical records of eligible patients were reviewed to extract demographic data (patients’ sex and age) and operation-related data (final defect area, size of the harvested flap, number of perforators, source branch from the pedicle, elevation plane of the flap, recipient vessels, and method of donor-site repair). For each case, the surgical outcomes were evaluated in terms of the donor- and recipient-site complications and the postoperative shoulder function, measured by the range of motion (ROM). Oncologic outcomes with local recurrences were also noted. Local recurrence was determined with histologic confirmation of the new growth of malignant neoplasm located within 2 cm of the primary wide excision site.
We evaluated the overall aesthetic outcome of the flap and donor site at 6 months postoperatively using a Stresser’s grading system, which consists of five items: malposition, distortion, asymmetry, contour deformity, and scarring [23]. We utilized postoperative photographs of frontal, lateral, and oblique views to assess the outcomes. “Noticeable” flaws were scored as one point, “obvious” flaws were scored as 5 points, and “deforming” flaws were scored as 15 points, with 1 to 4 points being a good result, 5 to 14 being mediocre, and 15 or greater being poor. Zero points were considered as “perfect” outcomes. The first author and corresponding author scored the photographs independently in five categories and then averaged the two scores to arrive at the final result.
During the follow-up outpatient visit, the active ROM of the patient’s shoulder was assessed by physical examination: shoulder extension, adduction, and internal rotation. No passive support was applied during shoulder movements. Patients were defined as having limitations if they felt pain, discomfort, or tightness in the joint area during the examination and were classified as having full ROM if none of these were present.
A total of 11 patients who underwent reconstruction with TDAP free flaps following ablation of malignant scalp neoplasms were included in the study. All but one of the patients were male; the average age was 72.6 years (range, 55–86 years) at the time of the surgery. The causes of the defect were primary cutaneous angiosarcoma in eight cases, recurrent angiosarcoma in two cases, and cutaneous squamous cell carcinoma in one case. The mean definitive defect size after completion of further excision, if necessary, was 169.7 cm2 (range, 120.0–220.0 cm2), with the bed of all raw surfaces being the bone layer of the calvarium. Five patients had local recurrences during the study period and were managed in a timely manner for those lesions (Table 1).
The final flap size, designed to address the defect area and the depth and contours of the surrounding soft tissues, averaged 223.2 cm2 (range, 160.0–406.0 cm2). The flaps were based on an average of 2.4 perforators (range, 1–4 perforators), with two source vessels (transverse and descending branches of the thoracodorsal artery) used in nine cases and one source vessel (descending branch of the thoracodorsal artery) used in two cases. The mean pedicle length was 10.0 cm (range, 8–13 cm). Six and five flaps were elevated above the deep fascia of the LD muscle and above the Scarpa’s fascia, respectively. The recipient vessels were obtained from the superficial temporal artery and vein on the defect side (n=10 cases) or the occipital artery and vein (n=1). The donor site was repaired with primary closure in all cases.
No total or partial flap losses occurred, and the flap survival rate was 100%. One patient underwent emergent reoperation on postoperative day 1 due to acute flap ischemia, and the flap was salvaged successfully. However, distal tip necrosis of approximately 2 cm2 occurred thereafter, although it completely healed by secondary intention later on. The mean length of hospitalization was 8.5 days (range, 6–14 days). All flaps enabled stable resurfacing for the scalp defects, an acceptable contour without the need for a secondary debulking operation, and satisfactory color matching with the adjacent skin.
Donor-site complications included a small amount of seroma in one case that was resolved by aspiration in the outpatient clinic and overt wound dehiscence in one case that was completely repaired using a split-thickness skin graft. The average period until complete stitch removal was 14.6 days (range, 9–25 days). At the 2-month follow-up, all patients were observed to have achieved full ROM of the ipsilateral donor shoulder. The aesthetic outcome of the flap and donor was in the “good result” category for all five items, with mean scores of 1.0 (range, 0.5–1.0) for malposition, 2.3 (range, 1.0–4.0) for distortion, 2.5 (range, 1.0–5.0) for asymmetry, 2.4 (range, 1.0–5.0) for contour deformity, and 1.2 (range, 0.5–2.5) for scarring.
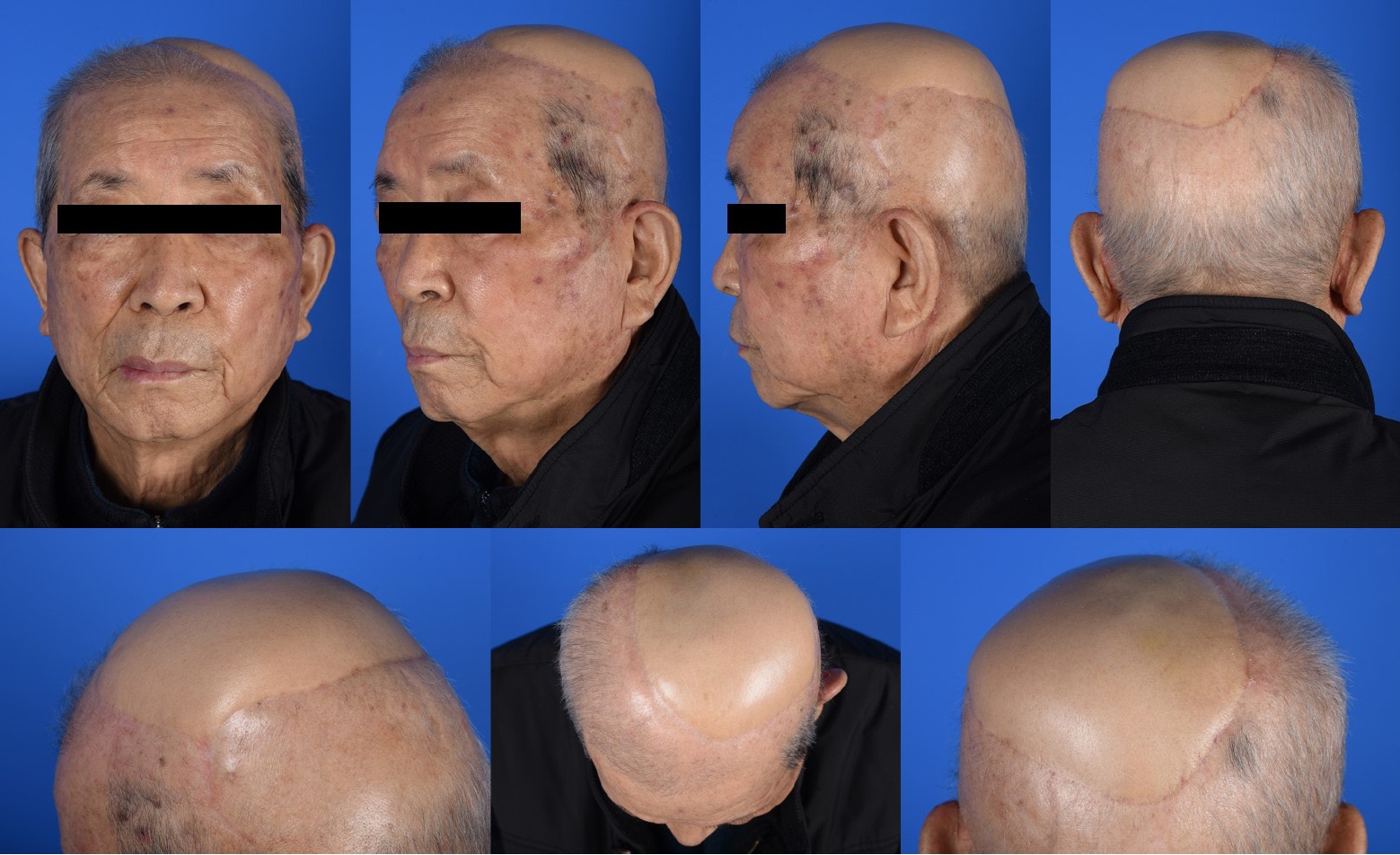
An 82-year-old man was referred to our center with a scalp biopsy finding of a primary cutaneous angiosarcoma. Preoperative imaging and systemic examination did not reveal any distant metastases; thus, wide excision of the angiosarcoma and coverage of the subsequent defect with free tissue transfer were planned. After marking the outermost border as far as it could be determined as a diffuse infiltrative lesion, a subperiosteal excision was performed on the tumor with a safety margin of approximately 2 to 3 cm. Intraoperative frozen section examination revealed a positive margin between 9 and 12 o’clock. After further resection, the final defect size was extensive at 15×11 cm2 (Fig. 1). A TDAP flap was designed with a 19.5×13.5 cm2 skin paddle; it included perforators from the descending and transverse branches of the thoracodorsal artery. The flap was harvested above the deep fascia. The recipient’s vessels were end-to-end anastomosed using superficial temporal vessels, and the donor site was restored with primary closure.
The patient was discharged on postoperative day 9 without flap compromise and other acute complications. No reconstruction-related complications occurred over the 1-year follow-up. The patient was satisfied with the cosmetic outcomes of the flap and did not experience functional shoulder impairment or limited ROM (Figs. 2, 3).
We have described our experience with using TDAP flaps as the primary choice for reconstructing large scalp defects after tumor ablation in 11 cases. With the exception of arterial insufficiency in one case, which was managed with timely intervention, all flaps were successfully transferred without perfusion-related complications and survived completely. We employed the TDAP flap, which offered extensive skin coverage up to 28×14.5 cm (406 cm2), as well as reduced donor site morbidity due to its muscle-sparing perforator flap nature. During postoperative follow-up, the patients showed acceptable aesthetic and functional outcomes without significant donor site- or recipient site-related complications.
In this case series, all but one case of squamous cell carcinoma had angiosarcoma as the origin of the scalp defect. Although five patients had findings of local recurrence within the study period, all of them were diagnosed with cutaneous angiosarcoma, which was likely due to the aggressive nature of the primary disease. Nevertheless, we achieved negative resection margins at the time of initial wide excision for all patients, indicating that the initial lesions were completely removed. The aggressive nature of the malignant neoplasm necessitates wide local excision, which inevitably results in an extensive raw surface. However, the scalp anatomy includes the galea aponeurosis and periosteum; these lack elasticity and are tight and immovable, thereby making local flap repair of large scalp defects difficult. Using skin grafts alone for defect coverage not only causes contour deformity but also requires a long time to be engrafted because the grafts use a bone bed in a periosteum-deficient environment. Therefore, microsurgical reconstruction could play a vital role in these circumstances, and as representative workhorse flaps, free LD musculocutaneous flaps and ALT fasciocutaneous flaps are most widely employed. Free radial forearm flaps are also considered as alternatives.
Reconstruction of extensive scalp defects using free LD musculocutaneous flaps has been well documented [8,9,13,14]. Their reliable blood supply, ease of harvesting, and provision of well-vascularized muscle are compelling to reconstructive surgeons; the operative outcomes are acceptable as well. However, its drawbacks include donor-site morbidity at the expense of LD muscle. A previous systematic review by our institution revealed that functional shoulder impairment may develop after LD muscle transfer, especially in the early postoperative period, and shoulder strength would weaken with shoulder extension, internal rotation, and adduction [15]. Conversely, muscle-sparing LD and TDAP flaps that left little-to-no amount of LD muscle were associated with a lower functional decline. Another drawback of LD flaps is that when combined with split-thickness skin grafts, wound healing is more delayed than that achieved with other flap-only modalities. From an oncologic standpoint, this can delay adjuvant radiation therapy, making it difficult to cure the primary cancer. However, reconstruction with TDAP flaps does not have these concerns, since it promotes rapid wound healing through dermis-to-dermis closure.
The advantages of ALT fasciocutaneous free flaps included a long pedicle length, minimal donor-site morbidity, and constant anatomy during flap elevation. Chou et al. [5] introduced their experience with reconstruction of scalp angiosarcoma using ALT free flaps; however, the dimension of the defect that could be covered was not that large, ranging from 4×2 cm2 to 8×13 cm2. As is well known, the vastus lateralis muscle can be used as a muscle flap. However, compared to LD, it has a limited size and amount of available soft tissue; furthermore, it has the disadvantage of requiring an additional skin graft [24,25]. Radial forearm free flaps are another alternative due to their long pedicle, pliable skin, and ease of elevation, however, their restricted size constrains their usage in cases wherein extensive skin paddles may be needed.
In this context, it is imperative to note the role of the TDAP flaps, which are thin, pliable, and muscle-sparing perforator flaps that cover a generous dimension [10,18,19]. Their main asset is that they allow for extensive resurfacing. We have prioritized these flaps as the primary surgical option to meet the reconstructive needs for a wide range of scalp defects. The flap width was maximized by including two source branches from the thoracodorsal artery whenever possible, and intra-flap anastomosis was performed to ensure flap perfusion when technically feasible. Furthermore, the TDAP flaps were customized with the following techniques to achieve favorable cosmetic outcomes at the recipient site and preserve function at the donor site. To ensure that the flap conformed to the thickness and curvature of the surrounding scalp, the flap thickness was tailored to the defect’s environment by adjusting the flap elevation plane, and the underlying LD muscle was not incorporated to minimize the donor-site morbidity. We also attempted to minimize the wound healing duration by maximizing the contact between the dermis of the transferred flap and the surrounding dermis at the recipient site. Notably, because most cases involved older adult patients with a wide range of underlying comorbidities, we ensured that the pedicles of the flaps were free from atherosclerotic changes, which facilitated microvascular anastomosis. Furthermore, oncologic origin defects frequently lack viable recipient vessels as a result of prior surgical procedures and radiation therapy. In such instances, the TDAP flaps proved advantageous by providing adequately long pedicles. Although we did not use the TDAP flaps as composite flaps containing different types of tissues, these flaps can serve as chimeric flaps as well. We summarized the comparison between the TDAP flaps and conventional flaps used for scalp defect reconstruction in Table 2.
The disadvantages of the TDAP flaps include the topographic variation in perforators. However, we used a high-frequency ultrasound device for meticulous perforator mapping since the middle of the study, which helped assess the exact subcutaneous course and configuration of the perforators. As indicated by the results of the present study, it was feasible to achieve primary closure in all cases. None of the patients experienced major donor-site morbidity, represented by long-term uncontrolled seroma or limited shoulder motion. The flaps survived with minimal risk in all cases. Thus, reconstruction of an extensive scalp defect using a TDAP flap may be advantageous in that it allows for primary closure with reduced functional impairment, resulting in decreased donor-site morbidity and rapid wound healing with dermis-to-dermis closure at the recipient site.
Several limitations should be noted in this study. The retrospective design and small sample size made the accurate interpretation of our findings and study outcomes difficult. Further, objective evaluation of the postoperative shoulder function was not carried out, and a quantitative assessment might be warranted. In order to solidify the results of this study, it would be beneficial to conduct future well-designed prospective studies with a larger sample size, directly comparing TDAP flap with other reconstructive approaches.
Fig. 1.
Patient number 5. (A) A final scalp defect measuring 15×11 cm2 is noted following the ablation of a primary angiosarcoma in the subperiosteal layer. (B) A large transverse thoracodorsal artery perforator (TDAP) flap measuring 19.5×13.5 cm2 is designed based on two branches of the thoracodorsal artery. (C) An elevated TDAP flap with a 9-cm-long pedicle. (D) Appearance after the final inset of the TDAP flap with generous dimensions. (E) The donor site is seen to have undergone primary repair.

Fig. 3.
Patient number 5. A linear scar, favorable in terms of aesthetic outcomes, is observed at the donor site.

Table 1.
Patient demographics and operation-related data
ROM, range of motion; M, male, F, female, STA/V, superficial temporal artery and vein; OA/V, occipital artery and vein; NPWT, negative-pressure wound therapy; Postop, postoperative; (R), recipient site; (D), donor site; NE, no event.
a)“Descending” refers to the descending branch of the thoracodorsal artery, and “transverse” refers to the transverse branch of the thoracodorsal artery.
Table 2.
Comparison of the TDAP flap to conventional flaps for scalp defect reconstruction
References
1. Jang HU, Choi YW. Scalp reconstruction: a 10-year experience. Arch Craniofac Surg. 2020;21:237-43.




2. Newman MI, Hanasono MM, Disa JJ, Cordeiro PG, Mehrara BJ. Scalp reconstruction: a 15-year experience. Ann Plast Surg. 2004;52:501-6.


3. Lim SY, Pyon JK, Mun GH, Bang SI, Oh KS. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-2.


4. Alharbi A, Kim YC, AlShomer F, Choi JW. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:e4827.



5. Chou PY, Kao D, Denadai R, Huang CY, Lin CH, Lin CH. Anterolateral thigh free flaps for the reconstruction of scalp angiosarcoma: 18-year experience in Chang Gung memorial hospital. J Plast Reconstr Aesthet Surg. 2019;72:1900-8.


6. Lutz BS, Wei FC, Chen HC, Lin CH, Wei CY. Reconstruction of scalp defects with free flaps in 30 cases. Br J Plast Surg. 1998;51:186-90.


7. Lee KT, Wiraatmadja ES, Mun GH. Free latissimus dorsi muscle-chimeric thoracodorsal artery perforator flaps for reconstruction of complicated defects: does muscle still have a place in the domain of perforator flaps? Ann Plast Surg. 2015;74:565-72.


8. Oh SJ, Lee J, Cha J, Jeon MK, Koh SH, Chung CH. Free-flap reconstruction of the scalp: donor selection and outcome. J Craniofac Surg. 2011;22:974-7.


9. Chang KP, Lai CH, Chang CH, Lin CL, Lai CS, Lin SD. Free flap options for reconstruction of complicated scalp and calvarial defects: report of a series of cases and literature review. Microsurgery. 2010;30:13-8.


10. Scaglioni MF, Giunta G. Reconstruction of cranioplasty using the thoracodorsal artery perforator (TDAP) flap: a case series. Microsurgery. 2019;39:207-14.



11. Kim SW, Hwang KT, Kim JD, Kim YH. Reconstruction of postinfected scalp defects using latissimus dorsi perforator and myocutaneous free flaps. J Craniofac Surg. 2012;23:1615-9.


12. Choi JH, Ahn KC, Chang H, Minn KW, Jin US, Kim BJ. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.




13. Lipa JE, Butler CE. Enhancing the outcome of free latissimus dorsi muscle flap reconstruction of scalp defects. Head Neck. 2004;26:46-53.


14. Hierner R, van Loon J, Goffin J, van Calenbergh F. Free latissimus dorsi flap transfer for subtotal scalp and cranium defect reconstruction: report of 7 cases. Microsurgery. 2007;27:425-8.


15. Lee KT, Mun GH. A systematic review of functional donor-site morbidity after latissimus dorsi muscle transfer. Plast Reconstr Surg. 2014;134:303-14.


16. Angrigiani C, Grilli D, Siebert J. Latissimus dorsi musculocutaneous flap without muscle. Plast Reconstr Surg. 1995;96:1608-14.


17. Hwang JH, Lim SY, Pyon JK, Bang SI, Oh KS, Mun GH. Reliable harvesting of a large thoracodorsal artery perforator flap with emphasis on perforator number and spacing. Plast Reconstr Surg. 2011;128:140e-150e.


18. Ayhan S, Tuncer S, Demir Y, Kandal S. Thoracodorsal artery perforator flap: a versatile alternative for various soft tissue defects. J Reconstr Microsurg. 2008;24:285-93.


19. Homsy C, Theunissen T, Sadeghi A. The thoracodorsal artery perforator flap: a powerful tool in breast reconstruction. Plast Reconstr Surg. 2022;150:755-61.


20. Shustef E, Kazlouskaya V, Prieto VG, Ivan D, Aung PP. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-25.


21. Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992;27:241-8.


22. Lee SH, Mun GH. Transverse thoracodorsal artery perforator flaps: experience with 31 free flaps. J Plast Reconstr Aesthet Surg. 2008;61:372-9.


23. Strasser EJ. Application of an objective grading system for the evaluation of cosmetic surgical results. Plast Reconstr Surg. 2002;109:1733-40.