 |
 |
- Search
| Arch Hand Microsurg > Volume 27(4); 2022 > Article |
|
Abstract
Purpose
Wide excision and subsequent reconstruction of the defect are crucial pillars in the treatment of soft tissue sarcoma (STS); however, those procedures carry a relatively high risk of postoperative complications, which could raise oncologic concerns. The present study evaluated the association of postoperative complications after resection and immediate reconstruction with STS recurrence.
Methods
We reviewed patients with primary STS who underwent wide resection and immediate reconstruction between 2011 and 2019. Patients were categorized into three groups based on their postoperative inflammatory complication status: no complications, noninflammatory complications, and inflammatory complications. Inflammatory complications were defined as those involving a sustained elevation of inflammatory markers in laboratory tests after postoperative 2 weeks. The cumulative incidence of oncologic events and their respective hazard ratios (HRs) were evaluated using multivariable Cox regression analyses.
Results
In total, 94 patients with a median follow-up of 54.8 months were analyzed, including 17 with inflammatory complications, 17 with noninflammatory complications, and 60 with no complications. The three groups showed similar baseline characteristics except for older age and a lower rate of FNCLCC (Fédération Nationale des Centres de Lutte Contre Le Cancer) grade 3 in the inflammatory complications group. The inflammatory complications group showed significantly worse disease-free survival than the no complications group. This difference remained significant after adjustment for other variables in multivariate analyses (HR, 3.485; p=0.019). The development of noninflammatory complications was not associated with oncologic outcomes.
Soft tissue sarcoma (STS), a heterogeneous subtype of malignant tumors consisting of more than 100 different histologic subtypes, displays a range of invasive potential and different oncologic prognoses [1,2]. Although it is difficult to generalize, surgical excision with a sufficient safety margin and solid reconstruction using various modalities are the mainstay of treatment. In a majority of cases, the resulting soft tissue defects may be closed primarily; however, those following excision of a large tumor or having insufficient soft tissue near the defect could raise the need for the reconstruction of soft tissue. In those cases, surgical reconstruction plays an essential role in providing adequate functional coverage of defects [3].
Although soft tissue reconstruction can provide effective local tumor control while facilitating limb salvage, it has inherent drawbacks including prolonged operation time, increased procedural complexity and wider spanned surgical fields, which may increase the risks of postoperative complications. In addition to the burden of the complication itself, there has been a concern regarding whether its development may lead to increased risks for the recurrence of original cancer [4]. Therefore, numerous studies have reported the potential association of postoperative complications with an increased risk of recurrence of gastric, colorectal, and breast cancer [5-7]. The suggestive mechanism, though not fully elucidated, is that inflammatory response caused by the postoperative complications could influence local residual and/or dormant tumor cells via inflammatory mediators, eventually triggering tumor recurrence [6,8-11].
In this regard, a few studies have investigated the potential association between postoperative complications and oncologic outcomes in STS as well [12-14]. However, they provided conflicting results, with one showing no correlation [12] but the other two suggesting postoperative complications as an independent predictor of disease-specific survival [13,14]. These inconsistent results might be attributable to a lack of classifying the postoperative complications according to the presence of systemic inflammation, the proposed mechanism for other primary malignancies [6,8-11].
Therefore, here we hypothesized that inflammatory postoperative complications were associated with STS recurrence, while noninflammatory ones were not. To test this hypothesis, this study evaluated the association between postoperative complications and oncologic outcomes in patients with STS who underwent wide resection and immediate reconstruction, with dividing the complications according to accompanying by systemic inflammation or not.
Ethics statement: This study was conducted after obtaining approval from the Institutional Review Board of Samsung Medical Center (No. 2022-07-176). The study was performed in accordance with the Declaration of Helsinki, and written informed consent was waived due to its retrospective nature.
We enrolled patients with primary STS who underwent wide resection and immediate reconstruction between January 2011 and December 2019. Patients were excluded if they underwent delayed reconstruction or distant metastasis was observed at the time of the first diagnosis.
Each patient’s case and treatment policy was discussed by our institution’s multidisciplinary sarcoma tumor board comprising surgeons, diagnostic radiologists, pathologists, medical oncologists, and radiation oncologists. The resection procedures were most commonly performed by orthopedic surgeons. Reconstructive procedures were performed by plastic surgeons, and the reconstruction modality was determined by the attending surgeon considering the patient’s general health, preference, defect location, and defect size. Postoperative sarcoma surveillance was mostly undertaken by orthopedic surgeons and by a medical oncologist when neoadjuvant or adjuvant chemotherapy and/or radiotherapy were required. Physical examinations and imaging workups, such as computed tomography or magnetic resonance angiography, were performed every 3 months for the first year postoperative, every 6 months thereafter until postoperative 5 years.
Patient demographic data and clinical outcomes were gathered from a prospectively maintained database and electronic medical records. Patient characteristics including age, sex, body mass index (BMI), comorbidities (hypertension and diabetes), smoking status, and American Society of Anesthesiologists physical status (ASA PS) classification at the time of surgery were collected. Tumor characteristics included location, histologic subtype, size, and Fédération Nationale des Centres de Lutte Contre Le Cancer (FNCLCC) histologic grade. Tumor size was classified according to the T (size) classification of the American Joint Committee on Cancer guidelines, 8th ed. Operation-related characteristics included margin status after final resection and the type of reconstruction procedure (local flap, free flap, or skin graft). In addition, data on whether neoadjuvant or adjuvant chemotherapy and/or neoadjuvant or adjuvant radiotherapy was administered were collected. Patients with hypertension were defined as those taking antihypertensive medication at the time of surgery. Diabetes patients were defined as those taking oral diabetes medication and/or insulin at the time of surgery. Smokers were defined as active smokers at the time of surgery.
The development of postoperative complications in the reconstructed area and the presence of sustained systemic inflammation were also assessed. Postoperative complications included an emergency return to the operating room due to a threatened flap, total flap or graft loss, partial flap or graft loss, delayed wound healing, seroma, venous thrombosis, and infection. Dehiscence was defined as an open wound requiring surgical debridement. Seroma was defined as fluid accumulation requiring aspiration and/or revision surgery. Venous thrombosis comprised postoperative deep vein thrombosis caused by a thrombus in the legs as well as pulmonary embolism. Infection was defined as the requirement for oral or intravenous antibiotics and/or surgical irrigation and debridement within 6 months of immediate reconstruction. If the C-reactive protein (CRP) level was not elevated or the infection was restricted to the reconstructed area, it was classified as a local infection, whereas if the CRP level increased or the infection spread to another part of the body, it was classified as a systemic infection. Among patients who developed postoperative complications, those with elevated CRP levels (>1.0 mg/dL) in laboratory tests until 2 weeks postoperative [6] were defined as having inflammatory postoperative complications. Patients were categorized into three groups based on the development of postoperative complications, with or without systemic inflammation: no complications, noninflammatory complications, and inflammatory complications.
The primary outcomes of interest were prognostic outcomes, which were identified from medical records. Prognostic outcomes including recurrence date and type (local and/or regional node and distant), date of the last follow-up, and disease status at that time were documented. The final follow-up date was that on which the patient had last visited the orthopedic surgeon or medical oncologist’s outpatient clinic for cancer surveillance or the date of death. Disease-free survival (DFS) was calculated as the time interval from the date of the first diagnosis to any type of the first occurrence of disease progression or death.
The baseline characteristics were compared between groups categorized by the development of postoperative inflammatory complications. Pearson chi-square test or Fisher exact test was used to analyzing categorical variables. Continuous variables were analyzed using the Mann-Whitney U-test, one-way analysis of variance, or the Kruskal-Wallis test. Survival curves for DFS were calculated and presented using the Kaplan-Meier method, and differences among the postoperative inflammatory complication groups were calculated using the log-rank test. For the DFS curve, STS recurrence and death during follow-up were analyzed. Patients were censored if relevant events did not occur during the follow-up period. Uni- and multivariate Cox regression analyses were performed to identify independent prognostic factors for oncological outcomes, with results expressed as hazard ratios (HR) and 95% confidence intervals (CI). A backward selection model was used for the multivariate analyses. The p-values <0.05 were considered statistically significant. All statistical analyses were conducted using IBM SPSS Statistics ver. 27.0 (IBM Corp., Armonk, NY, USA).
During the study period, 129 STS patients underwent wide resection and immediate reconstruction. After the application of the exclusion criteria, 94 patients were included in the analysis. The baseline characteristics of the study population are summarized in Table 1. The mean patient age was 50.8 years (range, 8–80 years), and the mean BMI was 25.3 kg/m2 (range, 16.9–38.45 kg/m2). Of the patients, 67.0% were male; the most common tumor location was the lower extremity (40 of 94, 42.6%). The local flap (46.8%) was the most commonly used reconstruction modality, followed by the free flap (37.2%). The median follow-up period was 54.8 months.
Among the 94 patients included in this study, 34 developed postoperative complications at the reconstructed site, of which delayed wound healing (12.4%) was the most common, followed by partial flap loss or skin graft loss (9.6%) and seroma (9.6%) (Table 2). Of those developing postoperative complications, 17 showed sustained elevation of inflammatory markers after postoperative 2 weeks, assigned as the inflammatory complications group, and the other showed no elevation of the markers, assigned as the noninflammatory group. In comparison of baseline characteristics among the three groups, the mean age and comorbidities (hypertension and diabetes) differed significantly (Table 1). The ASA PS classes at the time of surgery were distributed differentially, with a higher proportion of class II and III patients in the inflammatory complications group (64.7% vs. 41.2% vs. 38.3%). The operation-related characteristics were not significantly different across groups except for FNCLCC grade, which showed a lower rate of grade 3 in the inflammatory complications group (5.9% vs. 17.6% vs. 18.3%). The final margin status was negative in all cases of the inflammatory complications group. The rate of patients receiving neoadjuvant radiotherapy or adjuvant radiotherapy was highest in the noninflammatory complications group. Other characteristics were not significantly different among groups.
Table 3 shows the oncological outcomes of the patients at the first STS recurrence event. Overall, 17 patients developed postoperative STS recurrence. Local recurrence and regional lymph node recurrence occurred in seven (7.4%) and two cases (2.1%), respectively. One patient (1.1%) developed simultaneous locoregional recurrence, while seven (7.4%) were diagnosed with distant metastasis during the follow-up period. Eight patients (8.5%) died during follow-up, including six of disease progression (5.8%) and two of an unspecified cause. Of the 17 patients who developed inflammatory complications, six (35.3%) were diagnosed with postoperative recurrence. The recurrence rate in this group was higher than that among patients who developed noninflammatory complications (11.8%) or no complications (15.0%), but the difference was not statistically significant (p=0.157). The rates of local or distant recurrence were comparable among the three groups.
In the Kaplan-Meier analysis, the inflammatory complications groups showed inferior DFS compared to the control, which difference was marginally significant (p=0.071) (Fig. 1). The noninflammatory complications group and no complications group showed similar DFS (p=0.781). In the multivariable Cox regression analysis adjusted for other variables, the development of postoperative inflammatory complications was associated with inferior DFS versus that of patients with no complications (HR, 3.485; 95% CI, 1.226–9.901; adjusted p=0.019) (Table 4). In addition, neoadjuvant chemotherapy had a significant influence on DFS after the adjustment for other variables. However, the development of noninflammatory complications was not an independent predictor of DFS.
The present study investigated the potential association of a sustained systemic inflammatory state with postoperative complications on the STS prognosis of 94 patients who underwent the resection of STS and immediate reconstruction. Although the majority of patients included in previous studies were those in whom primary closure was possible [13,14], this study focused on the oncologic outcomes of patients requiring soft tissue reconstruction who tended to have challenging defects and thus had a high tendency to develop postoperative complications. We further classified their postoperative complications according to systemic inflammation status represented by CRP levels. Our study results are significant in that this is the first study to investigate the association between postoperative inflammatory complications and the oncologic prognosis of STS.
Similar to the previous studies, the most common location of the STS was the lower extremity (42.6%) [2]. The 5-year DFS rate of our study population (80.2%) with a median follow-up of 54.8 months is at least comparable to that of previous studies showing 5-year local recurrence-free survival rate of 74.2% [14] and 5-year overall survival rate of 79.9% (95% CI, 77.7%–82.1%) [15]. A slightly superior outcome of this study might be attributable to tumor size, as the majority in this study were <5 cm in diameter. In addition, as the plastic surgery team at our institution provided solid reconstructive support to optimize the challenging defect with various reconstruction options, all patients included in this study were able to undergo radical resection with sufficiently wide negative margins by the oncologic surgeon, enabling complete local tumor control.
Our data suggest that only sustained inflammatory complications of the reconstructed area were independent predictors of DFS in STS patients, while noninflammatory complications had no significant influence on STS recurrence. The association between the postoperative elevated CRP level and worse prognosis was investigated in a few studies of other gastric malignancies [16,17], and the findings of our study were analogous to those studies in that only inflammatory complications were prognostic factors in STS patients. Recent studies reported that a modified Glasgow prognostic score reflecting a high CRP level in the preoperative setting of STS patients might have reliable prognostic value [18-20], and the increase in preoperative CRP level is known to be mainly associated with oncologic conditions [18]. Whereas, postoperative CRP levels are considered more useful for predicting prognosis since they reflect both oncological conditions and postoperative inflammatory responses [4,16]. The relationship between postoperative CRP levels and worse prognosis is also under investigation [17].
As previously stated, systemic inflammation represented by elevated CRP levels (>1.0 mg/dL) in laboratory tests until 2 weeks postoperative [6] in our study population might result from the complex interplay between other oncologic effects and the influence of postoperative complications. However, the rate of FNCLCC grade 3 tumors was lower in the inflammatory complications group than in the other groups, and no patients had a positive or unknown final margin status, demonstrating full ablation of the primary tumor. Meanwhile, the complication profiles in the inflammatory complications group showed more severe characteristics than those of the other two groups, which showed a higher rate of reexploration or total flap/split-thickness skin graft loss. Therefore, it can be assumed that the elevated CRP level is more likely a result of complications themselves rather than oncologic interactions, and the inflammatory responses as a sequel to postoperative complications might play a central role in accelerating STS recurrence. Nevertheless, it further needs to be verified since the exact causal relationship between elevated CRP levels and complications has not yet been investigated, as well as the fact that elevation of CRP might be an interaction of local residual or dormant tumor cells.
In addition to the presence of inflammatory complications, neoadjuvant chemotherapy was an independent prognostic factor in the multivariate analysis. Dadras et al. [14] also reported that neoadjuvant chemotherapy was a predictor of inferior disease-specific survival, but this was described as a result of confounding bias in patients with high-grade sarcoma. In our study, only two patients received neoadjuvant chemotherapy; thus, this correlation might be a result of the small sample size.
Our results suggest that patients who develop severe complications that may be associated with systemic inflammation may need to be monitored for any sign of recurrence with close follow-up. In addition, it might be better to implement timely active intervention in patients with postoperative complications before the transition to the systematic inflammatory state to improve oncologic prognosis. Ultimately, reconstructive surgeons might need to consider proper reconstruction modalities and postoperative management to reduce complications and identify an aggressive intervention method that promotes rapid healing rather than expecting secondary healing in the event of flap or graft loss.
This study has several limitations. First, it is inherently limited by its retrospective study design and the small number of cases; thus, further large-scale well-designed prospective studies are required to draw more concrete conclusions. Second, no further analysis was conducted on whether this inflammatory response represented by an elevated CRP level was due to the complication itself or by a complex interplay between other systematic conditions; thus, it will be necessary to further evaluate whether the elevated CRP level per se might affect the oncologic outcomes of STS patients. Third, other clinical variables might have acted as cofounders in STS patients; however, because of the heterogeneous nature of STS, it was difficult to consider all other biological and clinical variables. Finally, our study included a mean follow-up period of 56.5 months, but the recurrence rate of our population tends to be lower than that of the previous studies; therefore, further long-term follow-up studies are necessary to capture late recurrence cases.
The findings of the current retrospective study suggest that the development of inflammatory complications after STS resection and immediate reconstruction may be associated with inferior prognostic outcomes. Noninflammatory complications were not associated with STS recurrence. Given our findings, in patients with severe complications that may be associated with systemic inflammation, implementing active intervention promptly and close follow-up for any sign of recurrence might be needed. Although further studies are warranted to evaluate a clear causal relationship, these results may contribute to preoperative individualized planning, patient counseling, and postoperative oncologic surveillance.
Fig. 1.
Disease-free survival according to the development of postoperative inflammatory complications (Cx).
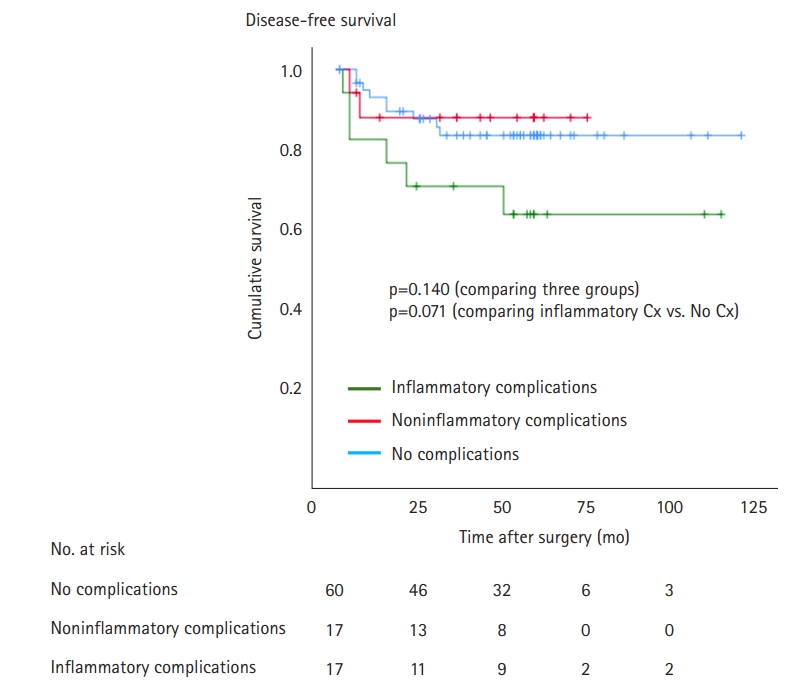
Table 1.
Comparison of baseline characteristics according to postoperative complication
Table 2.
Postoperative complication profile
Table 3.
Outcomes according to the presence of postoperative complications at the first event
Table 4.
Univariable and multivariable Cox regression analysis to identify the independent associations of each variable with disease-free survival
References
1. Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70:200-29.



2. Choi JH, Ro JY. The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Adv Anat Pathol. 2021;28:44-58.


3. Götzl R, Sterzinger S, Arkudas A, et al. The role of plastic reconstructive surgery in surgical therapy of soft tissue sarcomas. Cancers (Basel). 2020;12:3534.



4. Pucher PH, Aggarwal R, Qurashi M, Darzi A. Meta-analysis of the effect of postoperative in-hospital morbidity on long-term patient survival. Br J Surg. 2014;101:1499-508.



5. Katoh H, Yamashita K, Wang G, Sato T, Nakamura T, Watanabe M. Anastomotic leakage contributes to the risk for systemic recurrence in stage II colorectal cancer. J Gastrointest Surg. 2011;15:120-9.



6. Beecher SM, OʼLeary DP, McLaughlin R, Kerin MJ. The impact of surgical complications on cancer recurrence rates: a literature review. Oncol Res Treat. 2018;41:478-82.



7. Lee KT, Jung JH, Mun GH, et al. Influence of complications following total mastectomy and immediate reconstruction on breast cancer recurrence. Br J Surg. 2020;107:1154-62.



9. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-44.



10. Matsubara D, Arita T, Nakanishi M, et al. The impact of postoperative inflammation on recurrence in patients with colorectal cancer. Int J Clin Oncol. 2020;25:602-13.



11. Salvans S, Mayol X, Alonso S, et al. Postoperative peritoneal infection enhances migration and invasion capacities of tumor cells in vitro: an insight into the association between anastomotic leak and recurrence after surgery for colorectal cancer. Ann Surg. 2014;260:939-44.


12. Behnke NK, Alamanda VK, Song Y, et al. Does postoperative infection after soft tissue sarcoma resection affect oncologic outcomes? J Surg Oncol. 2014;109:415-20.



13. Broecker JS, Ethun CG, Monson DK, et al. The oncologic impact of postoperative complications following resection of truncal and extremity soft tissue sarcomas. Ann Surg Oncol. 2017;24:3574-86.



14. Dadras M, Koepp P, Wallner C, et al. Wound complications are a predictor of worse oncologic outcome in extremity soft tissue sarcomas. Surg Oncol. 2020;33:126-34.


15. Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17:671-80.


16. Kurokawa Y, Yamashita K, Kawabata R, et al. Prognostic value of postoperative C-reactive protein elevation versus complication occurrence: a multicenter validation study. Gastric Cancer. 2020;23:937-43.



17. Saito T, Kurokawa Y, Miyazaki Y, et al. Which is a more reliable indicator of survival after gastric cancer surgery: Postoperative complication occurrence or C-reactive protein elevation? J Surg Oncol. 2015;112:894-9.


18. Hou T, Guo T, Nie R, et al. The prognostic role of the preoperative systemic immune-inflammation index and high-sensitivity modified Glasgow prognostic score in patients after radical operation for soft tissue sarcoma. Eur J Surg Oncol. 2020;46:1496-502.









