|
 |
- Search
| Arch Hand Microsurg > Volume 27(3); 2022 > Article |
|
Abstract
End-to-end (ETE) and side-to-end (STE) anastomosis are two common configurations of lymphaticovenous anastomosis (LVA); however, it remains inconclusive which method is better. A 62-year-old man with lower extremity lymphedema underwent LVA with the STE method on the ankle. When the lymphatic vessel was cut for additional LVA at the proximal lower leg, blood drained out from the cut end of a lymphatic vessel, which suggested venous-lymphatic reflux at the STE anastomosis at the ankle. Because the reflux continued until 1 hour after the previous LVA at the ankle, the STE anastomosis at the ankle was re-explored and converted to ETE by ligation of the proximal lymphatic vessel. Reverse venous-lymphatic reflux was corrected, and a lymphovenous shunt was created immediately after the ligation. The current case suggests that STE anastomosis can be inferior to ETE anastomosis for creating a lymphovenous shunt when venous backflow exists.
Lymphaticovenous anastomosis (LVA) is technically challenging but becoming a popular treatment strategy for lymphedema since Koshima et al. [1] pioneered supermicrosurgery techniques. To create an effective lymphovenous shunt in LVA, pressure gradient from collecting lymphatic vessel to vein is fundamental. Therefore, an anastomosing functioning lymphatic vessel with high endolymphatic pressure to the reflux-free vein can be ideal [2]. However, various situations can be encountered in LVA, and finding reflux-free veins are not always possible [3,4].
A variety of LVA configurations exist, and each has its advantages and disadvantages [5,6]. End-to-end (ETE) anastomosis is the most common method that is less technically challenging but it can make only one-way bypasses. Although side-to-end (STE) anastomosis is technically demanding, it makes a bidirectional lymphatic bypass. STE LVA having bidirectional drainage without the need to ligate the proximal lymphatic vessel is theoretically superior to ETE LVA [5]. However, Yamamoto et al. [6] found that the occurrence of the venous backflow increased in the following order: ETE anastomosis, STE anastomosis, and ETS anastomosis. They concluded that ETE anastomosis, which has little backflow of venous blood into lymphatic channels, should be used because venous backflow into the lymphatics is considered to be related to the ineffectiveness of lymphovenous bypasses. On the other hand, Cheng et al. [5] reported greater efficacy by STE anastomosis compared to ETE. In this case report, a persistent retrograde venous-lymphatic reflux was observed after STE anastomosis at the lower extremity, which was not corrected during the surgery. The retrograde shunt was corrected by conversion of STE to ETE anastomosis by ligation of the proximal lymphatic vessel.
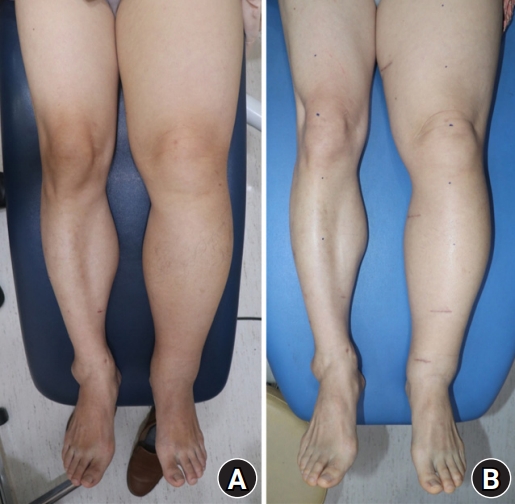
A 62-year-old male patient without specific medical history had suffered from progressive left lower leg swelling from 13 years ago. Two times of cellulitis episodes developed. Lymphoscintigraphy revealed lymphatic obstruction of the left lower extremity (Fig. 1). Indocyanine green fluorescence lymphography showed functional lymphatic vessels on the dorsum of the foot and ankle. Underdiagnosis of lymphedema unknown cause with International Society of Lymphology (ISL) stage II, LVAs were performed under local anesthesia in December 2019. First, STE anastomosis was performed at the ankle. The second incision site was on the proximal calf. In the meantime, bandage compression was not performed, and manual compression massage was performed on the foot intermittently. When the proximal lymphatic vessel was cut for additional LVA at the proximal calf, drainage of blood was observed from the cut edge of the lymphatic vessel (Fig. 2). The regurgitation of the blood through the lymphatic vessel remained until the completion of the LVA at the proximal lower leg. Therefore, the LVA site of the ankle was re-explored and venous-lymphatic reflux was observed at the STE anastomosis site. Additional veins with low venous pressure were searched for, but no additional veins were available within the incision site. We decided to perform conversion of the STE anastomosis to ETE anastomosis to increase the lymphatic pressure by ligation of the lymphatic vessel proximal to the anastomosis, which immediately resulted in a successful lymphovenous shunt. The venous-lymphatic reflux at the proximal calf also disappeared. A total of 3 ETE LVAs (medial ankle, proximal lower leg, and thigh) were performed. Postoperative outcome was analyzed by leg circumference change and patient-reported outcomes by a quality of life (QOL) measure for limb lymphedema (LYMQOL) questionnaire.
After 4 months, the average absolute circumference ratio difference decreased from 0.163±0.093 to 0.143±0.072 (Table 1, Fig. 3), and the overall QOL score on the LYMQOL questionnaire increased from 7 to 9 points. The patient’s subjective symptoms also improved. Before the operation, this patient complained of swelling and pain in the dorsum of the ankle. After one month, the pressure sense at the calf decreased and the skin became soft. After 4 months, the swelling of the foot was hardly felt and the pain disappeared. Cellulitis did not occur until 2 years of follow-up.
Written informed consent was obtained for the publication of this case report and accompanying images.
In this case report, venous-lymphatic reflux after the STE anastomosis was observed at the lymphatic vessel proximal to the anastomosis. This intraoperative finding suggested that the venous-lymphatic reflux was not temporary and might be continued after the surgery. The reverse flow after the LVA was reported to be a significant negative impact on the surgical outcomes of patients undergoing LVA [2]. The reverse venous-lymphatic reflux was successfully corrected by the conversion of the STE to ETE anastomosis. The current case showed that STE anastomosis might be inferior to creating lymphovenous shunt in selected cases.
STE anastomosis has two major advantages compared to ETE anastomosis in LVA. First, both antegrade and retrograde lymphatic flow can be drained to the venous flow. Second, original lymphatic flow can be preserved in case it does not have complete proximal obstruction. Therefore, STE anastomosis is one of the common anastomosis methods in LVA, and favorable outcomes have been reported [5]. However, as it was shown in this case, STE anastomosis showed a higher chance of retrograde venolymphatic flow. The retrograde venolymphatic flow in LVA can cause thrombosis in the anastomosis site and cause poor outcomes. In LVA surgery, endovascular pressure of the lymphatic should be higher than that of the vein to create lymphovenous shunt. In STE anastomosis, when an accumulation of lymphatic fluid in the collecting lymphatic vessel develops, endo lymphatic pressure can be decreased by the original lymphatic flow because the lymphatic pathway to a proximal lymphatic vessel is maintained. Therefore, retrograde venous-lymphatic flow can occur when strong venous backflow exists.
On the other hand, there is only one pathway in which accumulated lymphatic fluid can be drained in ETE anastomosis; anastomosed vein. In this case report, the retrograde venous-lymphatic flow was created after the STE anastomosis, and the reverse flow was confirmed in the proximal lymphatic vessels on the proximal calf. This result suggested that the retrograde shunt may not be corrected after the surgery. Intraoperative conversion of the STE to ETE anastomosis by ligation of proximal lymphatic vessel was reported in previous studies for management of retrograde venous-lymphatic flow in STE anastomosis in LVA [7,8].
Retrograde venous flow is found relatively frequently in patients with lymphedema. Chronic venous insufficiency is the entity most likely to complicate preexisting lymphedema [9]. There has been no studies which evaluated risk factors for venous insufficiency or increased venous pressure in patients with lymphedema. Finding reflux-free superficial veins using vein visualizer or high frequency ultrasonography can be effective ways to avoid retrograde venous-lymphatic flow in LVA [3,10].
The current case report was different from previous studies. First, the retrograde venous-lymphatic flow was confirmed outside of the LVA site. The blood flow was checked in another proximal lymphatic vessel, which suggested venous flow was shunted into the lymphatic system. The blood flow in the lymphatic system can increase the risk of thrombosis in other surgical sites of LVA. Second, the reverse venous-lymphatic flow was observed continuously during the surgery and was found not to be corrected. This observation suggested that the retrograde venous-lymphatic reflux might not be corrected after the surgery. In conclusion, the current case report suggested that STE anastomosis might be inferior to ETE anastomosis for creating lymphovenous shunt when strong venous backflow exists. Given that this is a report of a single case, further study might be necessary to clarify appropriate indications for performing STE anastomosis in LVA.
Fig. 1.
Preoperative lymphoscintigraphy. (A) Anterior and (B) posterior views. After injecting radiotracer into the subcutaneous area of the first and second interdigital spaces of both feet, whole-body imaging was performed at 1 hour. Lymphatic obstruction in the left lower extremity was revealed.
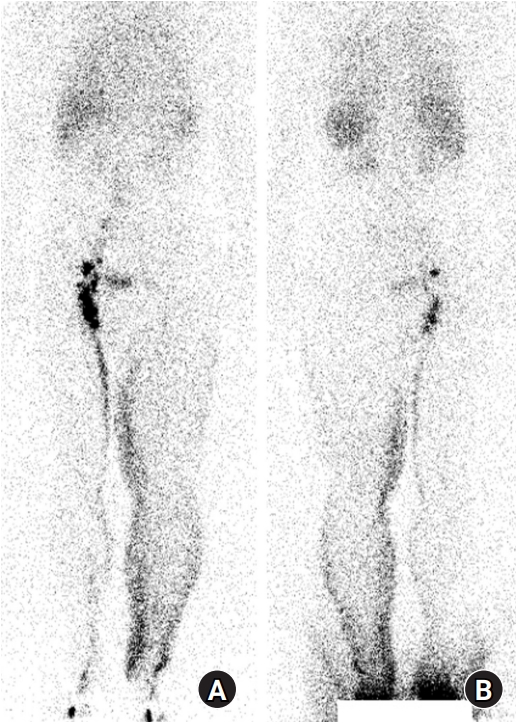
Fig. 2.
Intraoperative image. Yellow arrows indicate the lymphatic vessel, and red arrows indicate the vein. (A) Side-to-end (STE) lymphaticovenous anastomosis (LVA) at the ankle incision. The lymphatic vessel of the ankle incision is indicated by a yellow arrow. Lymphovenous shunt was not noted, and retrograde venous-lymphatic reflux was observed. (B) After about 30 minutes, venous flow through the cut end of the lymphatic vessel was detected in the proximal lower leg (yellow arrow). The venous outflow through the proximal lymphatic vessels continued and was not corrected during surgery. (C) The LVA site at the ankle was re-explored. Proximal end ligation of the lymphatic vessel on the ankle incision site was performed to convert STE into end-to-end anastomosis, which created a lymphovenous shunt. (D) Left lower leg showing the incision sites for LVA.

Table 1.
Serial circumference changes of the lower extremity
References
1. Koshima I, Inagawa K, Urushibara K, Moriguchi T. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg. 2000;16:437-42.


2. Visconti G, Salgarello M, Hayashi A. The recipient venule in supermicrosurgical lymphaticovenular anastomosis: flow dynamic classification and correlation with surgical outcomes. J Reconstr Microsurg. 2018;34:581-9.


3. Yang JC, Wu SC, Chiang MH, Lin WC. Targeting reflux-free veins with a vein visualizer to identify the ideal recipient vein preoperatively for optimal lymphaticovenous anastomosis in treating lymphedema. Plast Reconstr Surg. 2018;141:793-7.


4. Akita S, Yamaji Y, Tokumoto H, et al. Prevention of venous reflux with full utilization of venoplasty in lymphaticovenular anastomosis. J Plast Reconstr Aesthet Surg. 2020;73:537-43.


5. AlJindan FK, Lin CY, Cheng MH. Comparison of outcomes between side-to-end and end-to-end lymphovenous anastomoses for early-grade extremity Lymphedema. Plast Reconstr Surg. 2019;144:486-96.


6. Yamamoto T, Narushima M, Kikuchi K, et al. Lambda-shaped anastomosis with intravascular stenting method for safe and effective lymphaticovenular anastomosis. Plast Reconstr Surg. 2011;127:1987-92.


7. Suzuki Y, Sakuma H, Ihara J, Shimizu Y. Proximal ligation after the side-to-end anastomosis recovery technique for lymphaticovenous anastomosis. Arch Plast Surg. 2019;46:344-9.




8. Yang JC, Wu SC, Lin WC, Chiang MH, Hsieh CH. Reversing venous-lymphatic reflux following side-to-end lymphaticovenous anastomosis with ligation of the proximal lymphatic vessel. J Plast Reconstr Aesthet Surg. 2021;74:407-47.

9. Dean SM, Valenti E, Hock K, Leffler J, Compston A, Abraham WT. The clinical characteristics of lower extremity lymphedema in 440 patients. J Vasc Surg Venous Lymphat Disord. 2020;8:851-9.


10. Seki Y, Kajikawa A, Yamamoto T, Takeuchi T, Terashima T, Kurogi N. The dynamic-lymphaticovenular anastomosis method for breast cancer treatment-related lymphedema: Creation of functional lymphaticovenular anastomoses with use of preoperative dynamic ultrasonography. J Plast Reconstr Aesthet Surg. 2019;72:62-70.


-
METRICS

-
- 0 Crossref
- 1,258 View
- 30 Download
- Related articles in Arch Hand Microsurg
-
Lymphaticovenous Anastomosis in Lower Extremity Lymphedema: A Case Report2018 June;23(2)