 |
 |
- Search
| Arch Hand Microsurg > Volume 26(4); 2021 > Article |
|
Abstract
Purpose
This study was performed to assess the effect of prophylactic lymphovenous anastomosis on the prevention of arm lymphedema after axillary lymph node dissection for breast cancer treatment.
Methods
Among 69 women referred to undergo axillary lymph node dissection from January 2020 to June 2020, 21 were assigned to the treatment group and 48 to the control group. In the treatment group, 21 patients underwent prophylactic lymphovenous anastomosis for the prevention of breast cancer-related lymphedema. The other 48 patients in the control group did not undergo any preventive surgical treatment. Prophylactic lymphovenous anastomosis was performed at the same time as axillary lymph node dissection and breast cancer surgery. Postoperatively, all patients underwent circumferential measurements at 1, 3, and 6 months and lymphography at 6 months after the surgery.
Results
None of the patients in the treatment group had lymphedema after the surgery (0%). In the control group, lymphedema occurred in nine patients (18.8%, p=0.049). No significant differences in the arm circumference were observed in the treatment group during follow-up (p>0.05), whereas the arm circumference in the control group showed a significant increase at 1, 3, and 6 months after axillary lymph node dissection (p<0.05). There were no significant differences between the two groups in the arm circumference changes with respect to baseline at 1, 3, and 6 months after axillary lymph node dissection (p>0.05).
Breast cancer-related lymphedema (BCRL) is a progressive lifelong disease that affects approximately one-third of women who undergo breast cancer surgery with axillary lymph node dissection (ALND) [1]. BCRL is a debilitating condition that poorly influences the function, health, and quality of life [2]. The incidence of BCRL ranges from 9% to 42%; these wide variations depend on numerous factors, such as different methods of diagnosis, oncologic treatments, and different lengths of follow-up [1,3]. The time course for the development of lymphedema is variable and this condition rarely appears immediately after ALND. Most patients present with significant edema during the first 12 months following surgery [4,5].
Lymphedema is primarily treated with conservative care, such as the use of compression garments and rehabilitation treatment [6]. Curative treatment of lymphedema has proven difficult. Extravasation of proteins and fluid into the interstitium causes inflammation and tissue fibrosis, which results in lymphatic sclerosis, further inhibiting lymphatic circulation [7]. Thus, there is no generally accepted cure for lymphatic drainage dysfunction. Lymphovenous anastomosis (LVA), lymph node transfer, and stem cell therapy are the emerging treatment modalities for restoring lymphatic drainage. However, none of these treatments have proven to be curative. Therefore, the recent focus has shifted to risk reduction and prevention.
Over the past decade, advances in microsurgery have led to the increased use of LVA techniques to prevent BCRL. Prophylactic LVA can increase patients’ quality of life and decrease the clinical status of BCRL by preventing its occurrence after ALND. Thus, the aim of this study was to evaluate the effect of prophylactic LVA on preventing lymphedema after ALND at our institution.
This retrospective study included 69 women with breast cancer who were referred to undergo breast cancer surgery and ALND between January 2020 and June 2020. All patients with unilateral breast cancer underwent total ALND as a result of positive sentinel lymph node biopsy or axillary core needle biopsy using ultrasonography or radiographic examination. Of the 69 patients, 21 underwent prophylactic LVA at the same time as ALND, and these patients were classified as the treatment group. The other 48 patients who did not undergo prophylactic LVA during ALND were assigned to the control group. The patients were explained the purpose of the study and gave consent to access their clinical information and images. This study was approved by the Institutional Review Board of Pusan National University Hospital (No. 2106-025-104).
Measurement modalities to evaluate lymphedema in all the 69 patients included arm circumference measurements, lymphoscintigraphy (Tc-99m phytate, subcutaneous injection, both upper extremities), indocyanine green (ICG) (Diagnogreen injection, 2.5 mg/mL; Daiichi Pharmaceutical, Tokyo, Japan) lymphography, and bioelectrical impedance, carried out within 15 days before ALND. Based on lymphoscintigraphy and ICG lymphography, all patients had normal lymphatic flow and no lymphatic dysfunction before surgery. In addition, baseline patient characteristics, including age, weight, and height, as well as data on the clinical characteristics, such as the type of breast surgery and medical history were obtained.
Twenty-one patients in the treatment group underwent the prophylactic LVA technique performed by a single surgeon skilled in lymphatic microsurgery for the prevention of arm lymphedema after breast cancer surgery. The other 48 patients in the control group were not treated with prophylactic LVA or prophylactic compressive decongestive therapy (CDT) after breast cancer surgery.
One hour before the surgery, 0.4-mL ICG was injected subcutaneously into the first and third webspace of the hand and the medial and lateral borders of the volar surface of the wrist site. Breast cancer surgery (mastectomy or lumpectomy) was performed by three general surgeons under general anesthesia, followed by ALND. After the completion of the axillary dissection, the patients were referred to a plastic surgeon to undergo prophylactic LVA. For visual observation of the lymphatic channel, the lymphatic drainage channel of the axillary site was visualized using a near-infrared fluorescence camera (Moment K; IAN C&S, Seoul, Korea). The lymphatic drainage channel in the axillary region was visually confirmed, and the area where the flow of the lymphatic drainage channel was visible was roughly indicated using a marking pen. Based on the lymphatic drainage channel flow, 0.1 to 0.2 mL of indigo carmine dye was injected into the intradermal layer into the upper third of the arm. After injection, when a suitable vein was found, prophylactic LVA was performed using an end-to-end technique with nylon 11-0 interrupted sutures. The number of prophylactic LVA cases ranged from 1 to 2. The prophylactic LVA took approximately 30 to 60 minutes (Fig. 1). After performing prophylactic LVA, the patients were transferred from a plastic surgeon to a general surgeon, and wound closure was performed. When performing the Jackson Pratt drainage into the axillary region, attention must be paid to placing the drain tube so as not to damage the anastomosis. No additional treatments were provided, including drugs or rehabilitation therapies. There was no increase in the rate of hematoma, infection, or seroma compared to standard ALND. The passage of the patent carmine into the anastomosis vein was used to verify the patency of prophylactic LVA at the time of operation.
The patients received standard postoperative care for breast cancer surgery. Some patients received adjuvant radiotherapy or chemotherapy after the surgery. Physical therapy, compression bandage/stocking, and medication were administered to patients in both groups who had BCRL after ALND.
The 1-, 3-, and 6-months follow-up included circumferential measurements in all cases, and ICG lymphography was performed at the 6-month follow-up in all cases. All patients in the two groups were clinically evaluated after surgery using circumference measurements performed by a single plastic surgeon to reduce interobserver variability. This study followed a single-blinded process, where the assessor who measured the upper limb circumference was blinded to the group each patient belonged to. The measurements of both arms at three sites, including the wrist, 10 cm below, and 10 cm above the olecranon, were obtained using flexible tape (Fig. 2). Arm circumference measurements were defined as lymphedema if there was a difference of more than 2 cm from the contralateral limb. ICG lymphography images were classified from type I to V, depending on the ICG dermal backflow (DB) stage [8]. ICG DB stages I to V were defined as lymphedema (Fig. 3). All patients generally noticed increased arm circumferences, describing an increased stiffness or tenderness of soft tissues in a localized area, either in the wrist or forearm.
Values are presented as means±standard deviations or as percentages, when appropriate. Independent t-tests were performed to compare continuous variables. Fisher exact test or the chi-square test was performed to compare categorical variables. All data were analyzed using the R 4.0.1 IRR package (R Foundation for Statistical Computing, Vienna, Austria), and statistical significance was set at p<0.05. The differences in circumference between measurements before operation and those at 1, 3, and 6 months after LVA surgery in the two groups were compared using a paired t-test between timing. The comparison of arm circumference differences between the two groups over time was performed using repeated-measures analysis of variance.
The demographics and baseline characteristics of the patients are outlined in Table 1. There were no significant differences between the two groups in terms of the demographic data, location of the surgical site, number of lymph nodes retrieved, number of metastatic lymph nodes, type of breast surgery, radiotherapy, and chemotherapy (p>0.05).
As shown in Table 2, there was a significant difference in the lymphedema rate between the two groups (p=0.049). Furthermore, there were no cases of lymphedema development during 6 months among the 21 patients in the treatment group (Table 3). In contrast, in the control group, nine out of 48 patients (18.8%) developed lymphedema, with one patient who developed it at 1 month of follow-up (2.1%), two at 3 months of follow-up (4.17%), and six at 6 months of follow-up (12.50%). In all patients with lymphedema, the difference in bilateral arm circumference was more than 2 cm in at least one of the three measurement sites. With regard to the ICG DB stage in the control group, there were four patients with grade I (8.33%), three with grade II (6.25%), and two with grade III (4.17%).
Fig. 4 shows a graph illustrating the differences in the bilateral arm circumference according to the length of the follow-up period in the treatment and control groups. Fig. 4A shows the differences in circumferences of both arms according to the length of the follow-up period at 10 cm above the olecranon, Fig. 4B shows the respective result at 10 cm below the olecranon, and Fig. 4C shows the corresponding measurements at the wrist. In the treatment group, there were no significant differences in circumferences of both arms measured at the three sites compared to the corresponding preoperative values during the follow-up period (p>0.05). However, in the control group, the dimensions in postoperative differences in circumferences of both arms at the three sites were significantly higher compared to those measured preoperatively (p<0.05). When comparing the two groups over time, there were no significant differences in circumferences of both arms at the three sites (p>0.05).
In this study, we evaluated the effect of prophylactic LVA on the prevention of arm lymphedema in breast cancer patients after ALND. Most patients in our study had high-risk factors for lymphedema, undergoing ALND and adjuvant regional lymph node radiotherapy (76.8%). Most patients underwent adjuvant chemotherapy (78.3%). Although controversial, this risk factor has been independently associated with lymphedema development in some studies [9-11]. None of the patients in the treatment group were diagnosed with BCRL (0%). As observed from this result, there is a promising prospect of successful prevention of lymphedema in patients with high-risk factors for developing lymphedema by performing prophylactic LVA; and we argue that this finding presents an opportunity to expand the scope of applying preventive LVA in other patient groups at high risk for lymphedema as well as in breast cancer patients.
Nevertheless, the wide variability in the prevalence of cancer-related arm lymphedema and the rate of lymphedema are high. Sentinel lymph node biopsy was performed to prevent cancer-related lymphedema, but recent studies have argued that with sentinel lymph node biopsy alone, lymphedema rates cannot be ignored [12,13]. Thus, prevention is a key factor in avoiding lymphedema. In this study, the dissection of lymph nodes or lymphatics responding to the gamma-ray detector was performed while performing ALND by injecting technetium-99 radiolabeled colloid into the subcutaneous fat around the breast mass at four positions at 3, 6, 9, and 12 o’clock. The preservation status of the lymphatics or lymph nodes in the arm was not confirmed. If the lymphatic pathway from the arm could be visualized and confirmed using the axillary reverse mapping technique introduced in recent studies [14,15] and avoided during lymph node dissection, more arm lymphatic drainage could be preserved. However, as discussed by Boccardo [16], even when ALND was performed using the axillary reverse mapping technique, it was almost impossible to identify efferent lymphatics that truly preserved the lymphatic flow in the arm, because arm lymphatic drainage combines the common lymphatic pathways draining the breasts. Therefore, since preservation is practically impossible, in this study, prophylactic LVA was performed at the same time as ALND.
Many LVA shunting procedures have been developed. In this study, prophylactic LVA was performed using supermicrosurgical LVA, and none of the 21 patients who underwent surgical prevention developed lymphedema (0%). This rate was lower than that of 4.34% in primary prevention of arm lymphedema using the telescopic LVA technique presented by Boccardo [16]. However, since this study only presents a follow-up period of 6 months, which is shorter than the follow-up period in Boccardo’s research, it is necessary to perform further evaluation with a longer period for an accurate comparison.
LVA with telescopic technique uses larger veins, which are a major cause of increased intraluminal pressure, which can lead to venous-lymphatic reflux and thrombosis [17]. Supermicrosurgical LVA aims to overcome this problem by anastomosing lymphatics and venules <0.8 mm in diameter in an intima-to-intima manner, which supposedly has a lower occlusion rate, by minimizing backflow and nonendothelial tissue in the vessel lumen [18]. In addition, a recent animal study revealed that supermicrosurgical LVA patency rates were higher than those of lymphovenous implantation [19]. Therefore, although this is a preliminary study, there was no patient who developed lymphedema among those who underwent supermicrosurgical LVA as the primary prevention; thus, it is expected that with a longer follow-up period and higher number of patients, the proposed supermicrosurgical LVA will establish itself as a competitive technique with a lower rate of lymphedema compared to telescopic LVA.
The disadvantage of supermicrosurgical LVA is that the operation time is longer than that of the telescopic LVA technique. In most of the previous studies, the supermicrosurgical LVA technique for lymphedema prevention was performed at heterotopic locations, which are different from those used in lymphadenectomy, and the operation time measured in these studies ranged from approximately 30 minutes to 2 hours [20,21]. In this study, LVA was performed in the axillary region where lymphadenectomy was executed to reduce the operation time, which is a weakness of the supermicrosurgical LVA technique; thus, LVA was performed in isotopic rather than in heterotopic locations. In this manner, the time taken for operation was approximately 30 minutes to 1 hour, which resulted in a reduction in the operation time by approximately 1 hour compared to performing LVA at heterotopic locations. Furthermore, there were no significant expenses for prophylactic LVA because it was immediately preceded after ALND and required only 30 to 60 minutes to perform. The microscope, microsurgical and supermicrosurgical tools, and surgeons were already available, and the cost of suture material was negligible.
Among the various methods for lymphedema diagnosis, the measurement of arm circumference is the most common technique. Measuring the circumference of the arm is simple and convenient without the need for specific equipment or space, but it requires interobserver reliability that cannot be achieved. However, circumferential measurements have been found to be accurate and reliable when performed by trained staff [22]. Therefore, in this study, a single trained plastic surgeon performed these measurements in all patients to reduce interobserver variability as much as possible. In addition, ICG lymphography was performed in all patients at 6 months of follow-up, and imaging was done to assess the functions of the lymphatic drainage channels. The presence of DB was confirmed in all patients with differences in bilateral arm circumference of more than 2 cm; no DB was confirmed in patients with a respective difference of <2 cm, and they showed a linear lymphatic pattern.
Swelling that occurred in the arm on the site of oncological surgery in the first year is likely transient lymphedema [23]. The rate of transient lymphedema is approximately 10.8% to 71.0% [23-26]. However, each transient lymphedema study has a different way of measuring and defining the disease, leading to variability in the rate of occurrence [24].
The majority of postoperative transient lymphedema diagnoses resolved completely within 3 to 6 months of onset [24]. In the present study, the treatment group underwent oncological surgery, followed by prophylactic LVA. The evaluation of lymphedema was performed at 1, 3, and 6 months postoperatively, and the evaluation method included the measurements of the circumference of both arms and ICG lymphography. However, volume analysis or bioimpedance spectroscopy evaluation was not performed. None of the patients in the treatment group had >2 cm difference in the three areas of the arm circumference measured at 1, 3, and 6 months postoperatively; no DB was observed on ICG lymphography in any of the patients at 6 months postoperatively.
While we do not believe that the rate of transient lymphedema is zero in the treatment group, it is thought that the rate of arm swelling can certainly be reduced in the treatment group compared to the control group since primary prophylactic LVA was performed. The rate of transient lymphedema is 0, probably due to the small number of patients in the treatment group, or some patients may have undergone transient lymphedema, but swelling may not have occurred during the clinic visits after the surgery. It could also be that the transient lymphedema had occurred between the visits and resolved before the next visit. Other studies report a lower rate of lymphedema occurrence after the prophylactic lymphedema surgery compared to the typical rate of transient lymphedema [16,27,28]; therefore, it can be thought that the prophylactic lymphedema surgery lowers the rate of lymphedema.
The incidence rate of BCRL ranges from 3% to 65%, and this can be due to the surgical treatment approach, the diagnosis of lymphedema, and the duration of follow-up [29-31]. The occurrences of BCRL were 4.4%, 10.1%, 15.2%, 28.6%, 31.2%, and 32.5% at 1, 3, 6, 12, 18, and 24 months after surgery, respectively [32]. According to recent reports, a primary lymphatic insufficiency state was identified on ICG lymphography, which may explain an increased likelihood of lymphedema after oncological treatment in patients with the preexisting disease compared with their healthy counterparts [33]. Therefore, it is seen that the pathologic changes in the lymphatic vessels occur before the symptoms of lymphedema in patients who received oncological treatment. While transient lymphedema can occur in both control and treatment groups, the incidence of BCRL was 0% at 6 months postoperatively in the treatment group and 18.8% in the control group. This is similar to the rate of lymphedema reported in other studies and may be considered reliable [16,32]. Although the follow-up duration is short, a statistically significant difference in the rate of lymphedema was observed during the study periods, and this is thought to be sufficient to demonstrate the effects of prophylactic lymphedema surgery. Therefore, the fact that there is a difference in the rate of lymphedema clearly indicates that preventive LVA is effective in the prevention of lymphedema. If a patient had a difference in arm circumference measurements of >2 cm at the postoperative outpatient visit or if DB was observed on lymphography, it was determined that the patient developed lymphedema, and physical therapy and compression bandage/stocking were performed in these patients.
In the treatment group, there was a minimal difference in arm circumference measured at 1, 3, and 6 months postoperatively, but the difference in arm circumference in the control group gradually increased at 1, 3, and 6 months postoperatively. However, this difference between the two groups was not statistically significant over time. This study was a preliminary study, and variations in arm circumference were measured during the follow-up period of 6 months postoperatively. It is believed that the difference in arm circumference in the control group would increase with an increase in the length of the follow-up period. Thus, it is likely that the difference in arm circumference between the two groups over time will be statistically significant.
Different follow-up periods were considered when evaluating the effect of prophylactic LVA on BCRL. Some studies argued that a minimum follow-up period of 3 years after initiating breast cancer treatment should be considered to adequately detect patients at risk of developing postoperative lymphedema [5,34]. This preliminary study had 1-, 3-, and 6-months follow-up periods, with a mean follow-up period of less than 12 months. This follow-up period is considered relatively short because, in the majority of patients, lymphedema develops approximately during the first year after breast cancer treatment. However, this preliminary study made a significant contribution because it has demonstrated that prophylactic LVA significantly prevented the occurrence of lymphedema after ALND. Therefore, the authors contend that, based on the findings of this study, the effect of prophylactic LVA for the prevention of lymphedema can be clearly demonstrated if the length of the follow-up period is increased to 3 years or longer and the number of patients is sufficiently increased.
In this study, there was a difference in the number of patients between the two groups. The reasons for the greater number of patients in the control group included the patient’s refusal to undergo prophylactic LVA, a change in the surgery schedule, and sudden change in the plan to perform ALND. The sudden change in the plan to perform ALND occurred because the metastasis finding of the axillary lymph node was negative on core needle biopsy at outpatient visiting; however, the metastasis finding of the axillary region was positive on sentinel lymph node biopsy at operation after undergoing mastectomy or lumpectomy. Some of the patients lacked a clear understanding of lymphedema, or they thought it would not happen to them, and there was also a reluctance to undergo preventive surgery. This study demonstrated a positive effect of prophylactic LVA as the primary strategy for BCRL prevention in patients who were referred for a lymph node dissection. Therefore, providing active education to patients who will undergo ALND on lymphedema and providing them with appropriate knowledge on the effectiveness of prophylactic LVA for primary preservation of BCRL, can improve their quality of life by reducing the BCRL rate.
We demonstrated the effects of prophylactic LVA. Unfortunately, patients in the control group did not receive any prophylactic CDT before the diagnosis of lymphedema. Therefore, comparative studies between prophylactic CDT and prophylactic LVA for the prevention of BCRL are required.
There are some limitations to our study. First, although our study has powerful strengths, including multiple measurement methods of lymphedema, it was not designed as a randomized trial. Second, our study was limited by the number of patients in the treatment group for analysis. Third, our follow-up period was 6 months, which was a short time to evaluate the exact incidence of lymphedema.
Advanced and sophisticated treatments for breast cancer have allowed patients to survive longer. Therefore, improving the quality of life is becoming a priority. Surgery should be more conservative in trying to preserve function and reduce morbidity. Prophylactic LVA was shown to be an effective treatment strategy for lymphedema in patients who underwent ALND over a 6-month follow-up period. We look forward to continuing our studies among more diverse patient groups and over longer periods.
Fig. 1.
Prophylactic lymphovenous anastomosis (LVA) operative technique application. The images show anastomosis between the 0.3-mm lymphatic drainage vessel and the 0.4-mm vein using an end-to-end technique with nylon 11-0 interrupted sutures. (A) Prophylactic LVA operative technique applied on the axillary region. (B) Prophylactic LVA operative technique at microscopic magnification.
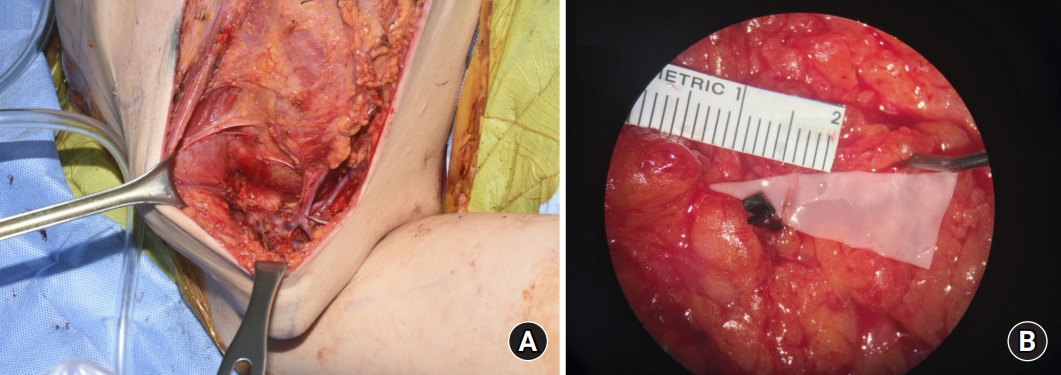
Fig. 2.
Locations of three areas in the upper limb. The upper extremity circumference is measured at the olecranon, 10 cm above and 10 cm below the olecranon, and at the wrist.
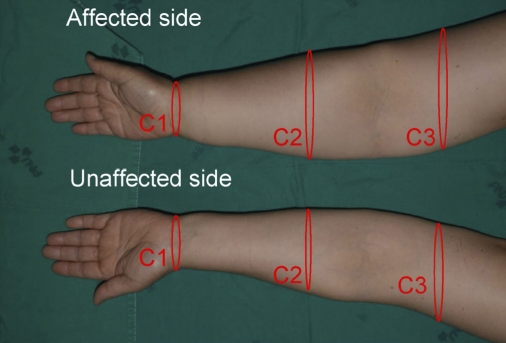
Fig. 3.
Lymphography performed at the 6-month follow-up after prophylactic lymphovenous anastomosis. Linear patterns in bilateral upper limbs are shown in lymphography (right, affected side; left, unaffected side).

Fig. 4.
Arm circumference differences at the preoperative (Pre), postoperative 1 month (F1), postoperative 3 months (F3), and postoperative 6 months (F6) in the treatment group and control group. Arm circumference differences are represented by the gradient (blue, treatment group; yellow, control group). (A) Arm circumference differences 10 cm above the olecranon with different follow-up periods. (B) Arm circumference differences 10 cm below the olecranon with different follow-up periods. (C) Arm circumference differences at the wrist with different follow-up periods.
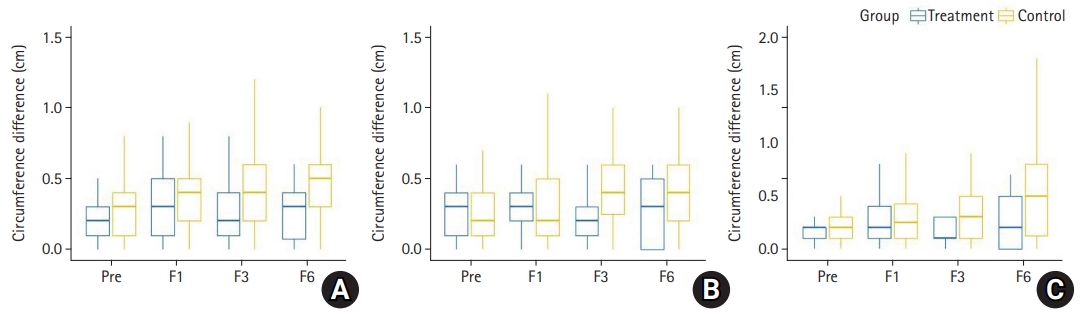
Table 1.
Patient characteristics
Table 2.
Comparison between the two groups according to lymphedema rates after axillary lymph node dissection
| Variable | Overall (n=69) | Treatment group (n =21) | Control group (n=48) | p-value |
|---|---|---|---|---|
| No. of patients | 0.049* | |||
| Undiagnosed with lymphedema | 61 (88.4) | 21 (100) | 39 (81.3) | |
| Diagnosed with lymphedema | 8 (11.6) | 0 (0) | 9 (18.8) |
Table 3.
Time of lymphedema onset and the ICG DB stage in both groups
REFERENCES
1. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500-15.


2. Carl HM, Walia G, Bello R, et al. Systematic review of the surgical treatment of extremity lymphedema. J Reconstr Microsurg. 2017;33:412-25.



3. Nguyen TT, Hoskin TL, Habermann EB, Cheville AL, Boughey JC. Breast cancer-related lymphedema risk is related to multidisciplinary treatment and not surgery alone: results from a large cohort study. Ann Surg Oncol. 2017;24:2972-80.




4. McDuff SG, Mina AI, Brunelle CL, et al. Timing of lymphedema after treatment for breast cancer: when are patients most at risk? Int J Radiat Oncol Biol Phys. 2019;103:62-70.


5. Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368-77.


6. Rogan S, Taeymans J, Luginbuehl H, Aebi M, Mahnig S, Gebruers N. Therapy modalities to reduce lymphoedema in female breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2016;159:1-14.



7. Hespe GE, Nores GG, Huang JJ, Mehrara BJ. Pathophysiology of lymphedema: is there a chance for medication treatment? J Surg Oncol. 2017;115:96-8.


8. Rasmussen JC, Tan IC, Marshall MV, et al. Human lymphatic architecture and dynamic transport imaged using near-infrared fluorescence. Transl Oncol. 2010;3:362-72.



9. Zhu W, Li D, Li X, et al. Association between adjuvant docetaxel-based chemotherapy and breast cancer-related lymphedema. Anticancer Drugs. 2017;28:350-5.


10. Hugenholtz-Wamsteker W, Robbeson C, Nijs J, Hoelen W, Meeus M. The effect of docetaxel on developing oedema in patients with breast cancer: a systematic review. Eur J Cancer Care (Engl). 2016;25:269-79.


11. Kim M, Kim SW, Lee SU, et al. A model to estimate the risk of breast cancer-related lymphedema: combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:498-503.


12. Sener SF, Winchester DJ, Martz CH, et al. Lymphedema after sentinel lymphadenectomy for breast carcinoma. Cancer. 2001;92:748-52.


13. Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491-500.



14. Thompson M, Korourian S, Henry-Tillman R, et al. Axillary reverse mapping (ARM): a new concept to identify and enhance lymphatic preservation. Ann Surg Oncol. 2007;14:1890-5.



15. Nos C, Lesieur B, Clough KB, Lecuru F. Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary dissection. Ann Surg Oncol. 2007;14:2490-6.



16. Boccardo FM, Casabona F, Friedman D, et al. Surgical prevention of arm lymphedema after breast cancer treatment. Ann Surg Oncol. 2011;18:2500-5.



17. Demirtas Y, Ozturk N, Yapici O, Topalan M. Supermicrosurgical lymphaticovenular anastomosis and lymphaticovenous implantation for treatment of unilateral lower extremity lymphedema. Microsurgery. 2009;29:609-18.


18. Tiwari P, Coriddi M, Salani R, Povoski SP. Breast and gynecologic cancer-related extremity lymphedema: a review of diagnostic modalities and management options. World J Surg Oncol. 2013;11:237.



19. Ishiura R, Yamamoto T, Saito T, Mito D, Iida T. Comparison of lymphovenous shunt methods in a rat model: supermicrosurgical lymphaticovenular anastomosis versus microsurgical lymphaticovenous implantation. Plast Reconstr Surg. 2017;139:1407-13.


20. Yamamoto T, Yamamoto N, Yamashita M, Furuya M, Hayashi A, Koshima I. Efferent lymphatic vessel anastomosis: supermicrosurgical efferent lymphatic vessel-to-venous anastomosis for the prophylactic treatment of subclinical lymphedema. Ann Plast Surg. 2016;76:424-7.


21. Takeishi M, Kojima M, Mori K, Kurihara K, Sasaki H. Primary intrapelvic lymphaticovenular anastomosis following lymph node dissection. Ann Plast Surg. 2006;57:300-4.


22. Armer JM, Stewart BR, Smith K, Cormier JN. Lymphedema following cancer treatment. In: Lester JL, Schmitt PA, editors. Cancer rehabilitation and survivorship: transdisciplinary approaches to personalized care. Pittsburgh, PA: Oncology Nursing Society; 2011. p. 133-43.
23. Kilbreath SL, Lee MJ, Refshauge KM, et al. Transient swelling versus lymphoedema in the first year following surgery for breast cancer. Support Care Cancer. 2013;21:2207-15.



24. Johnson AR, Fleishman A, Granoff MD, et al. Evaluating the impact of immediate lymphatic reconstruction for the surgical prevention of lymphedema. Plast Reconstr Surg. 2021;147:373e-381e.


25. Boccardo F, Casabona F, De Cian F, et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: over 4 years follow-up. Microsurgery. 2014;34:421-4.


26. Feldman S, Bansil H, Ascherman J, et al. Single institution experience with lymphatic microsurgical preventive healing approach (LYMPHA) for the primary prevention of lymphedema. Ann Surg Oncol. 2015;22:3296-301.



27. Casabona F, Bogliolo S, Valenzano Menada M, Sala P, Villa G, Ferrero S. Feasibility of axillary reverse mapping during sentinel lymph node biopsy in breast cancer patients. Ann Surg Oncol. 2009;16:2459-63.



28. Onoda S, Todokoro T, Hara H, Azuma S, Goto A. Minimally invasive multiple lymphaticovenular anastomosis at the ankle for the prevention of lower leg lymphedema. Microsurgery. 2014;34:372-6.


29. Gärtner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment: a nationwide study of prevalence and associated factors. Breast. 2010;19:506-15.


30. Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102:111-8.



31. Wernicke AG, Goodman RL, Turner BC, et al. A 10-year follow-up of treatment outcomes in patients with early stage breast cancer and clinically negative axillary nodes treated with tangential breast irradiation following sentinel lymph node dissection or axillary clearance. Breast Cancer Res Treat. 2011;125:893-902.



32. Zou L, Liu FH, Shen PP, et al. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: a 2-year follow-up prospective cohort study. Breast Cancer. 2018;25:309-14.



-
METRICS

-
- 1 Crossref
- 2,066 View
- 56 Download
- Related articles in Arch Hand Microsurg






